Description
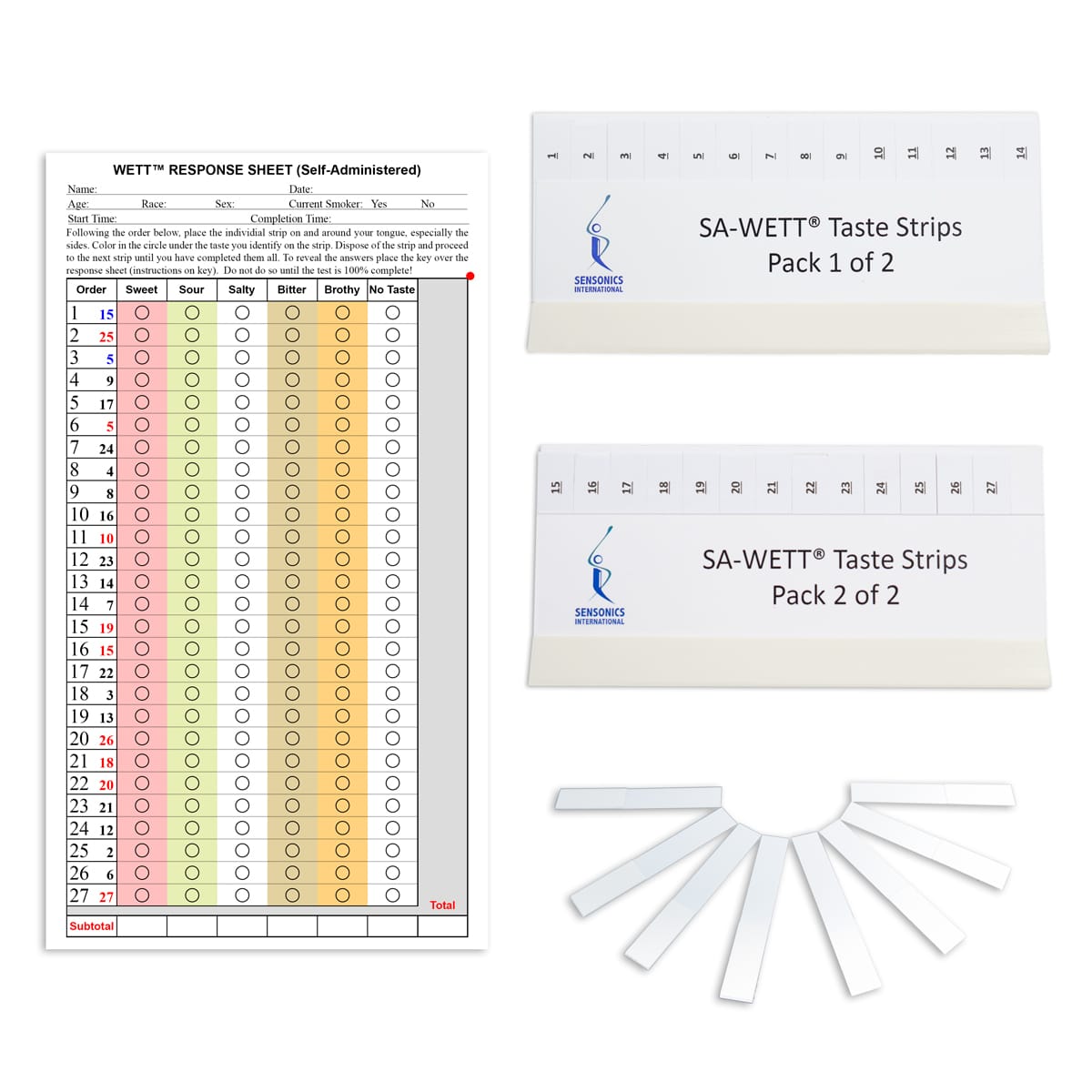
The Brief Self-Administered Waterless Empirical Taste Test™ (WETT®-SA-27)
This 27-stimulus version of the WETT® Taste Test for sense of taste on the taste buds can be sent through the mail to subjects for self-administration. Each individually packaged B-WETT kit comes with response forms and instructions. Ideal for situations where the full WETT® is impractical. Administration time is less than 10 minutes. CE certified.
Individually packaged kit with enclosed response forms. One scoring key included per order. Additional keys may be purchased. The Administration Manual is not included and must be purchased separately.
Why test for sense of taste of and sense of smell?
For all the right reasons!
- Quality of Life
- Personal Safety
- Health
- Nutrition
- Occupational Safety
- Early Intervention
The senses of smell and taste on the taste buds monitor the intake into the body of all nutrients and airborne chemicals required for life. These senses provide a warning for toxic fumes, smoke, leaking natural gas, spoiled food, and dangerous environments. Importantly, they determine the flavor and palatability of foods and beverages, and provide a vast array of aesthetic delights.
Unlike vision and hearing, smell and taste testing is not routinely performed by physicians, the government, the school system, or employers. This is in spite of the fact that decreased chemosensation, particularly smell loss, can be very debilitating, placing an individual, as well as coworkers, at risk in many occupations (e.g., chemical manufacturing, nuclear plant maintenance, fire fighting, police work, plumbing, aviation, nautical engineering and maintenance, and many military and industrial situations). Those who work in hazardous environments or in situations where smell warning is essential should be evaluated regularly. Such testing can be employed to transfer people with dysfunction to jobs in which decreased smell function has no major safety consequences. Smell loss can be an early sign of some diseases. Sensonics’ tests can be used to detect smell loss but cannot be used alone to diagnose disease.
These types of kits are often used by food manufacturers, restaurants, research organizations, and culinary professionals to gather valuable insights into consumer preferences, product quality, and market trends. They provide a structured and controlled way to compare and contrast different options, helping businesses make informed decisions and improve their offerings.
USFCR and ISO Certified
Sensonics International is a USFCR Verified Vendor and ISO certified 13485:2016. ISO 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Such organizations can be involved in one or more stages of the life-cycle, including design and development, production, storage and distribution, installation, or servicing of a medical device and design and development or provision of associated activities (e.g. technical support). ISO 13485:2016 can also be used by suppliers or external parties that provide product, including quality management system-related services to such organizations.
Requirements of ISO 13485:2016 are applicable to organizations regardless of their size and regardless of their type except where explicitly stated. Wherever requirements are specified as applying to medical devices, the requirements apply equally to associated services as supplied by the organization.
The processes required by ISO 13485:2016 that are applicable to the organization, but are not performed by the organization, are the responsibility of the organization and are accounted for in the organization’s quality management system by monitoring, maintaining, and controlling the processes.
If applicable regulatory requirements permit exclusions of design and development controls, this can be used as a justification for their exclusion from the quality management system. These regulatory requirements can provide alternative approaches that are to be addressed in the quality management system. It is the responsibility of the organization to ensure that claims of conformity to ISO 13485:2016 reflect any exclusion of design and development controls.
If any requirement in Clauses 6, 7 or 8 of ISO 13485:2016 is not applicable due to the activities undertaken by the organization or the nature of the medical device for which the quality management system is applied, the organization does not need to include such a requirement in its quality management system. For any clause that is determined to be not applicable, the organization records the justification as described in 4.2.2.